It goes without saying probably a majority of people want to find some sort of balance. Whether it’s in balance between work and social life, balance in a diet, or even literal balance (esp. if you’re a gymnast!), balance is probably something we all strive for!
Likewise, it’s not surprising that even chemical reactions also strive for a certain balance between their amount of reactants and products — this special state of balance in chemical reactions is referred to as equilibrium.
After covering this chapter overview, you’ll understand some of the basic concepts of equilibrium and will be prepared to tackle our more in depth articles. Let’s dive in!
Equilibrium on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try to approach general chemistry topics in equilibrium, similar to topics concerning redox reactions. While generally, there might not be individual questions covering equilibrium, take the concepts learned from this equilibrium and apply them to other general chemistry topics!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Equilibrium
As stated above, an important way to approach studying equilibrium is to use the concepts learned in this chapter overview and articles and apply them when learning other MCAT content material!
You’ll probably see the best overlap of equilibrium contents when you cover thermodynamics as these are 2 very related concepts and ideas!
1. Equilibrium of Chemical Reactions
Before talking about equilibrium, it’s best first to understand that reactions can be reversible. This means that while reactions usually proceed forward where reactants form products, they can also be reversed where the products form reactants.
There’s a certain point where the rate of the forward reaction (i.e. reactants ⇒ products) equals the rate of the reverse reaction (i.e. products ⇒ reactants) — at this point, the reaction is at equilibrium.
Another way to think about it is that at equilibrium, the concentrations of the reactants and products will remain constant. However, we have to make this distinction: it DOES NOT mean that the concentrations of the reactants and products are equal. Check out the figures below to have a better visual!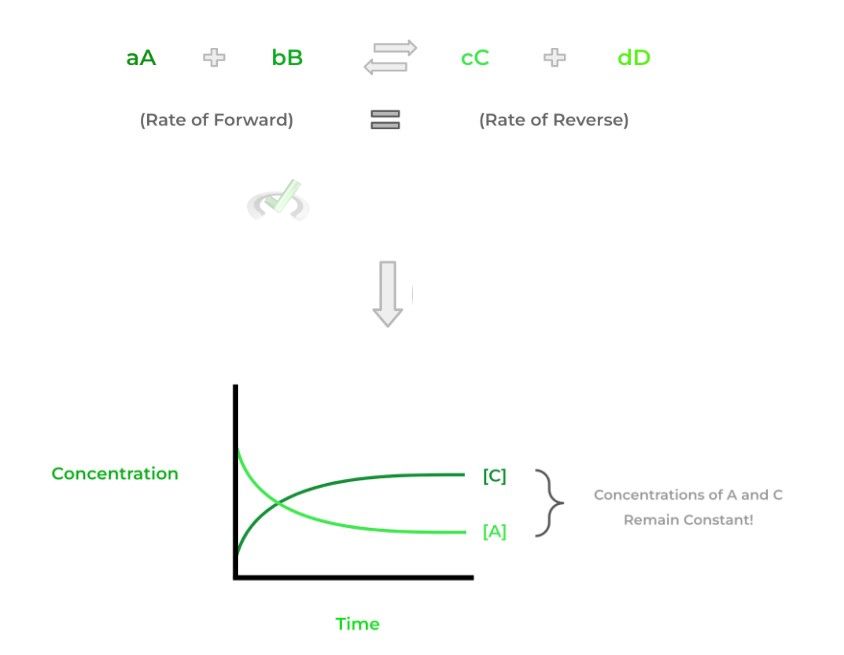
Notice how in the graph above, there’s a certain point where the concentrations of A and C remain constant (B and D) not shown. It’s at this point that equilibrium is achieved.
When talking about reaction equilibrium, we can actually put a number to this equilibrium state called the equilibrium constant (Keq). This can be calculated by the following ratio below:
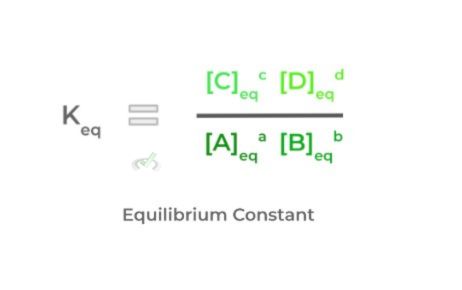
It’s important to note that the concentrations of the reactants and products utilized for the calculation are their concentrations when the reaction is at equilibrium — we tried to better emphasize this with the subscript! Also, notice how the stoichiometric coefficients become the exponent!
In addition to the equilibrium constant, there is a related value called the reaction quotient (Q). This is similar to the equilibrium constant, as the calculation setup is the same.
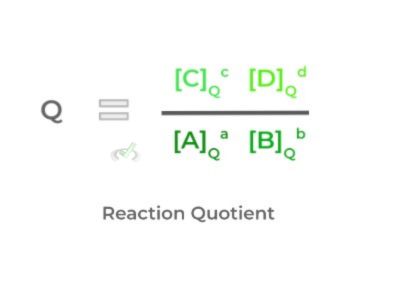
The major difference, however, is that the concentration values won’t necessarily be the same as those when in equilibrium – again, we tried to best indicate this by the subscript!
There are actually a couple of important relationships and conclusions that we can make when we compare the values of the equilibrium constant and reaction quotient. Take a look at the 3 relationships below and the rationale behind the conclusions we can get from them!
A. Q < Keq: Forward Reaction Favored
>> Why?
- A smaller Q value indicates that the denominator (i.e. the concentration of the reactants) value is very large indicating excess reactants
- The forward reaction is favored to form more products and reestablish equilibrium!
B. Q = Keq: Equilibrium Achieved
>> Why?
- This should be relatively straightforward as the concentrations of the reactants and products are the same for those in equilibrium!
C. Q > Keq: Reverse Reaction Favored
>> Why?
- This is essentially the reverse of point A! A larger Q value indicates the numerator (i.e. the concentrations of the products) value is large indicating excess products.
- The reverse reaction is favored to form more reactants and reestablish equilibrium!
(Coming Soon!) Full Study Notes : Equilibrium of Chemical Reactions
For more in-depth content review on equilibrium of chemical reactions, check out these detailed lesson notes created by top MCAT scorers.
2. Le Chatelier’s Principle
Don’t worry – you won’t have to worry about improving your French to pronounce this principle. You’ll just be responsible for how to apply its concept to MCAT discrete and passage problems come test day!
In its simplest form, Le Chatelier’s Principle states that when there is a disturbance in the chemical equilibrium of a reaction, the reaction will take the necessary steps in order to reestablish the equilibrium.
This will usually come in the form of changing the concentrations of the reactants/products. When doing these problems, try to get comfortable with the phrase “shift left/right” as we’ll introduce.
The best way to understand this principle is through some examples and doing multiple practice problems! Let’s again use the equation above!
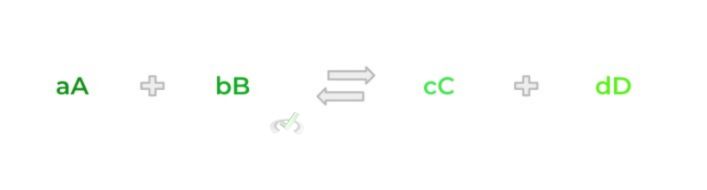
A “shift right” in the equation occurs when equilibrium is disturbed in a way where the resulting reaction quotient becomes less than the reaction equilibrium. This can be due to 2 changes in concentration:
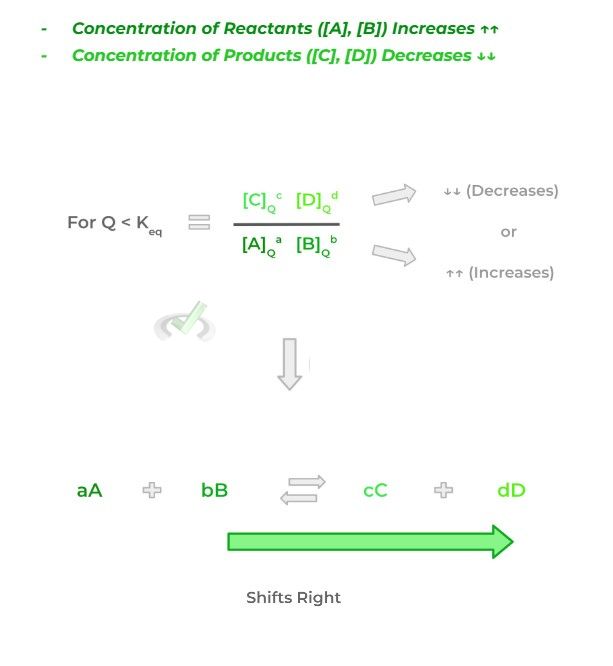
Thus, in order to reestablish the equilibrium, the most obvious way would be to increase the product concentration. Additionally, the concentrations of the reactants can decrease which is the answer that the MCAT will probably prefer.
A “shift left” in the equation occurs when equilibrium is disturbed in a way where the resulting reaction quotient becomes greater than the reaction equilibrium. This can be due to 2 changes in concentration:
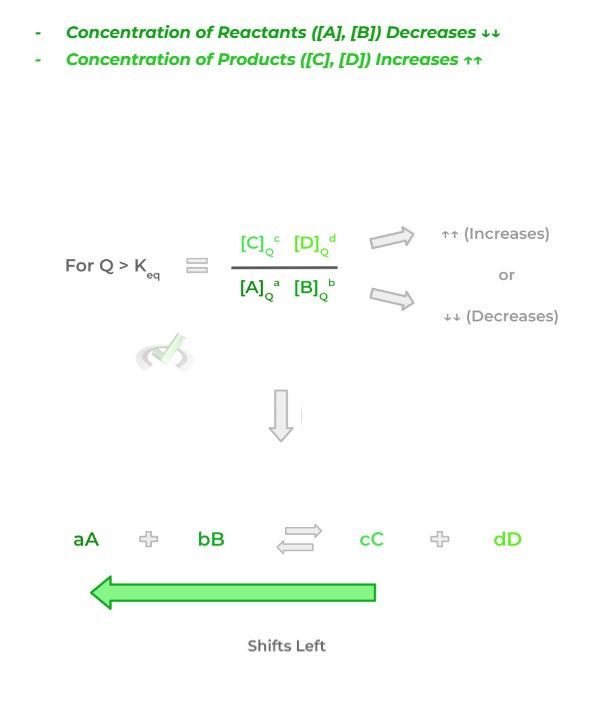
Likewise, in order to reestablish the equilibrium, the concentration of the reactants could increase, or the concentrations of the products could also decrease to compensate for the equilibrium shift.
A great way to think about this is like balancing a seesaw! Just like when a seesaw becomes unbalanced, you can either add or subtract mass on the sides of the seesaw to restore balance.
(Coming Soon!) Full Study Notes : Le Chatelier’s Principle
For more in-depth content review on applying Le Chatelier’s principle, check out these detailed lesson notes created by top MCAT scorers.
3. Kinetic v.s. Thermodynamic Product of Chemical Reactions
Though the title of the subsection might be a little intermediating at first, it can be easily understood when broken down! Additionally, you’ll often see that this concept will have the best application when put into the context of organic chemistry!
When given a certain set of reactants, different conditions can result in the formation of different products! Specifically, we’ll focus on whether the reaction is done at low or high temperatures.
Let’s first give an example of a reaction where 2 possible products can be formed. Take for example the deprotonation of 2-methylcyclohexanone by a strong base: 2 products can be formed because the base can deprotonate from either the 2 or 6 carbon.
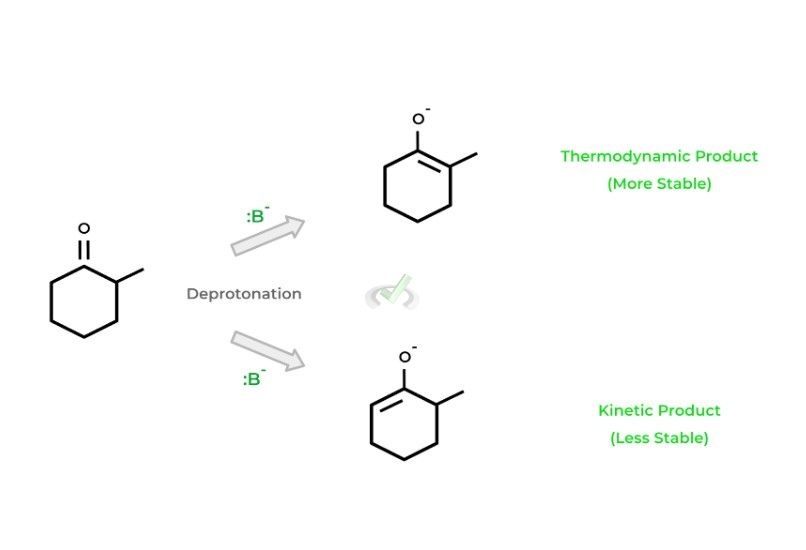
Note that the product formed from the deprotonation at carbon 2 is much more stable because of the methyl substituent!
The more stable one is called the thermodynamic product, while the less stable one is referred to as the kinetic product. It’s shown that the temperature in which the reaction occurs can influence which product is formed. Look at the relationships and rationales below:
Low Temperatures ⇒ Kinetic Product Formed
>> Why?
Because there is not enough heat to overcome the activation energy to form the thermodynamic product, the kinetic (less stable) product is formed as it requires much lower activation energy.
High Temperature ⇒ Thermodynamic Product Formed
>> Why?
Now, a sufficient amount of heat is present to overcome the activation energy to form the thermodynamic product.
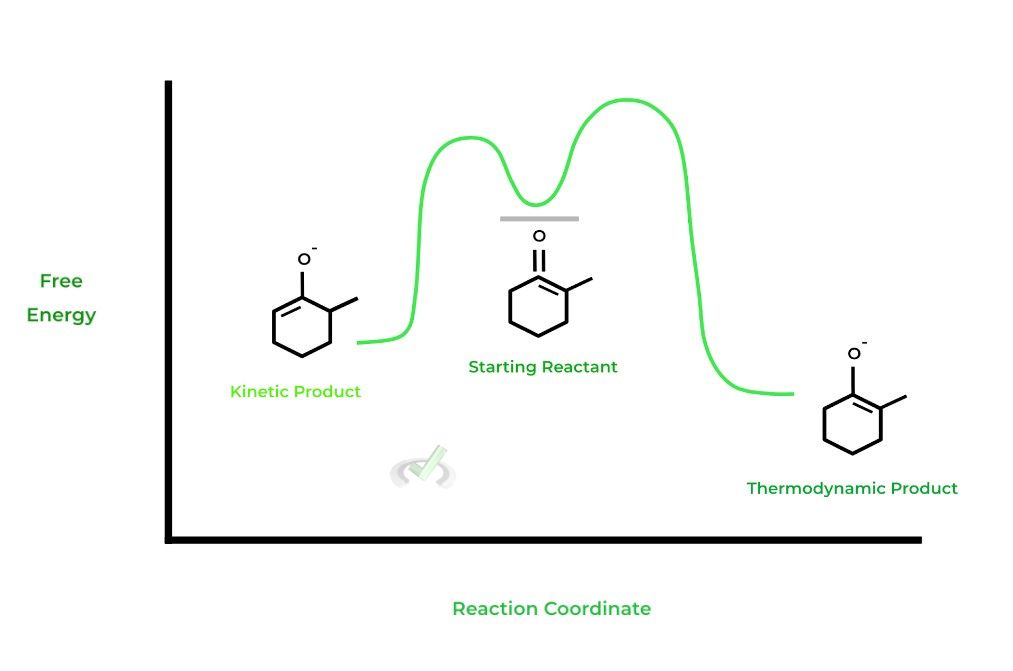
There are a couple of important things to note about the reaction coordinate diagram. Note that the thermodynamic product has a lower energy level compared to the kinetic product, which is why it’s more stable.
Additionally, notice again the difference in the activation energies when forming the kinetic and thermodynamic products!
(Coming Soon!) Full Study Notes : Kinetic v.s. Thermodynamic Product of Chemical Reactions
For more in-depth content review in understanding in regards to the difference between the kinetic and thermodynamic products., check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about equilibrium in general chemistry!
Term | Definition |
|---|---|
Equilibrium | Point in a reversible chemical reaction where the rate of the forward reaction equals the rate of the reverse reaction; Concentrations of reactants and products remain constant |
Equilibrium Constant | The ratio of the concentration of the products and the reactants when at equilibrium; The concentrations are raised to the power of their stoichiometric coefficients |
Reaction Quotient | The ratio of the concentrations of the products and reactants but will not necessarily be at equilibrium; Similarly, the concentrations are raised to the power of their stoichiometric coefficients |
Thermodynamic Product | One possible product of a reaction which is more stable and is favorable in high temperatures |
Kinetic Product | One possible product of a reaction which is less stable, but will be favorable in low temperatures |
Additional FAQs - Equilibrium on the MCAT
What is the Law of Mass Action? – MCAT
The same goes for the reverse reaction: the rate of the reverse reaction is equal to the product of the product concentrations (which in this case are the “reactants”) and the rate constant, raised to the power of their stoichiometric coefficients.
What are the 3 Characteristics of Equilibrium? – MCAT
What is Equilibrium State Law? – MCAT
Are Rate Laws on the MCAT?
Additional Reading Links (Coming Soon!) – Study Notes for Equilibrium on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Acids and Bases on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these