When you think of a textbook jump shot, you might think of Klay Thompson’s slick shooting form. For a textbook backhand in tennis, Serena William’s power and accuracy may come to mind. Similarly, carboxylic acids also have this “textbook” aura in organic chemistry!
As we’ll see in this chapter overview, carboxylic acids (and their derivatives) are textbook examples of the highly fundamental nucleophilic substitution reaction, arguably one of the most important reactions in organic chemistry.
This chapter overview will introduce you to the principal characteristics, nomenclature, and reactions of carboxylic acids and their derivatives! Let’s go!
Carboxylic Acids and Derivatives on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Compared to other tested organic chemistry topics, this will be one of the most emphasized. As such, be prepared for about 8-9 questions that might come up testing this topic.
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Carboxylic Acids and Derivatives
When reviewing carboxylic acid, piggyback off of the concepts and ideas you learned when studying the chemistry of aldehydes and ketones! A lot of the fundamentals learned there can be applied here as well!
Similar to other topics we’ve covered, we’ll focus on the generalities that will suffice in order for you to do well on the MCAT! Again, this won’t be your Ochem 1/2 midterm or final: the basics will do for your success on the MCAT!
1. Basic Structure and Nomenclature of Carboxylic Acids
Carboxylic acids are organic molecules characterized by the presence of a carboxyl group which contains a carbonyl group and a hydroxyl group bonded to the carbonyl carbon — hence the term, carboxylic acid.
Structurally, carboxylic acids are very similar to aldehydes: just replace the hydrogen atom with a hydroxyl group. Likewise, carboxylic acids are also located at the end of the organic molecule because of the hydroxyl group just like aldehydes!

Luckily when naming carboxylic acids, you won’t have to worry about prefixes because carboxylic acids are the highest priority functional group and will only have a suffix! Simply replace the “-ane” suffix of the corresponding alkane with “-oic acid”!

Due to the presence of the hydroxyl group, it’s evident that carboxylic acids participate in hydrogen bonding which increases their polarity. Also affecting their polarity is the carbonyl group as it is also polar due to the dipole moment generated.
Full Study Notes : Basic nomenclature, structure, and characteristics of carboxylic acids
For more in-depth content review on basic nomenclature, structure, and characteristics of carboxylic acids, check out these detailed lesson notes created by top MCAT scorers.
2. Nucleophilic Substitution Reactions of Carboxylic Acids
Carboxylic acids have very similar carbonyl chemistry and nucleophilic reactions with aldehydes and ketones! However, notice instead of writing a nucleophilic addition reaction, we wrote nucleophilic substitution reaction for carboxylic acids.
This is because, with carboxylic acids, we now have a possible leaving group in the form of the hydroxyl group attached to the carbonyl carbon (more on that coming up). Contrast this to aldehydes and ketones where there aren’t very good leaving groups.
Below is a common nucleophilic substitution mechanism for carboxylic acid, specifically called an addition-elimination mechanism. A nucleophile attacks the electrophilic carbon and “adds” to the molecule to create a tetrahedral intermediate. When the carbonyl is reformed, the leaving group is “eliminated”.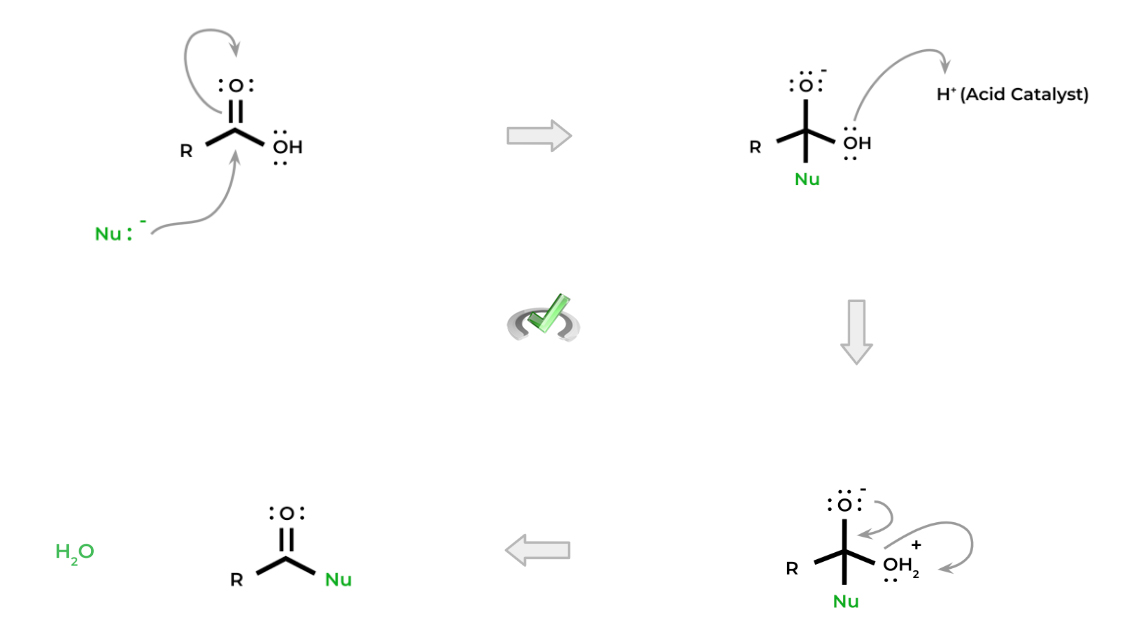
There are a couple of important things to note: first, notice how the alcohol is protonated via the acid catalyst. This is important because the hydroxyl group by itself is not a good leaving group.
When protonated, the hydroxyl group becomes a water molecule which is a much better leaving group! This acid catalysis is important precisely because of this reason — though the reactions can be catalyzed by a base, we’ll cover the various nucleophilic substitutions of carboxylic acids primarily utilizing an acid catalyst.
Finally, notice how also the carbonyl is also able to be reformed! This is also due to the fact that we now have a leaving group that we can eliminate now. Let’s now cover 3 basic nucleophilic substitution reactions!
One of the most fundamental nucleophilic substitution reactions for carboxylic acids is esterification. As the name implies, we form an ester functional group which can be accomplished with an acid catalyzed nucleophilic substitution with an alcohol!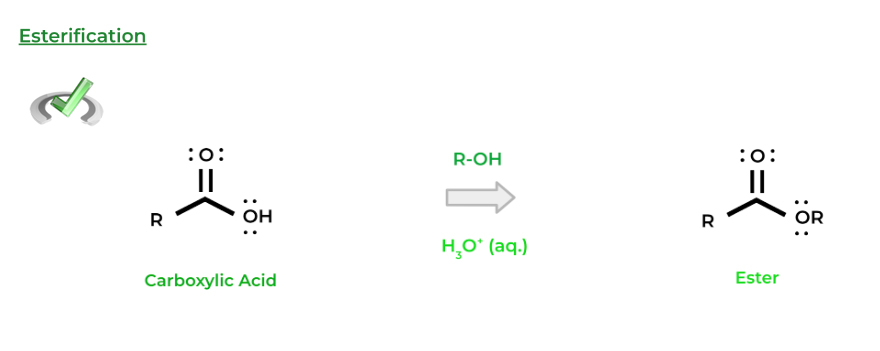
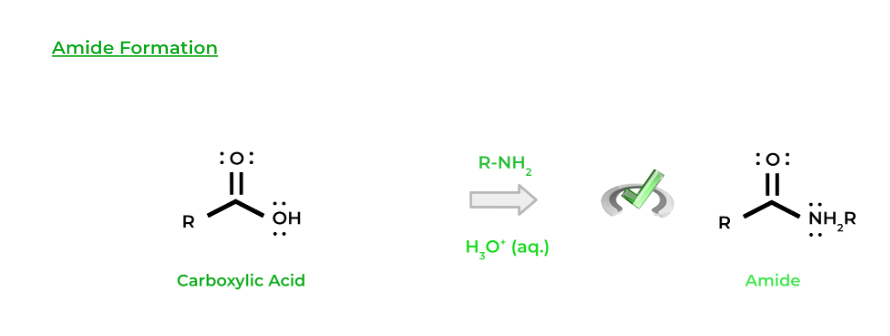
Similarly, another type of organic molecule that can be formed is an amide, where an amine can function as a nucleophile. Just like esterification, we’ll use an acid catalyst in order to convert the hydroxyl group into a better leaving group.
Finally, carboxylic acids can also react with each other to form molecules called anhydrides. In this case, the hydroxyl group of one carboxylic acid can act as the nucleophile and attack another carboxylic acid! While we can always use an acid catalyst, heat can also just be applied for this reaction to occur!
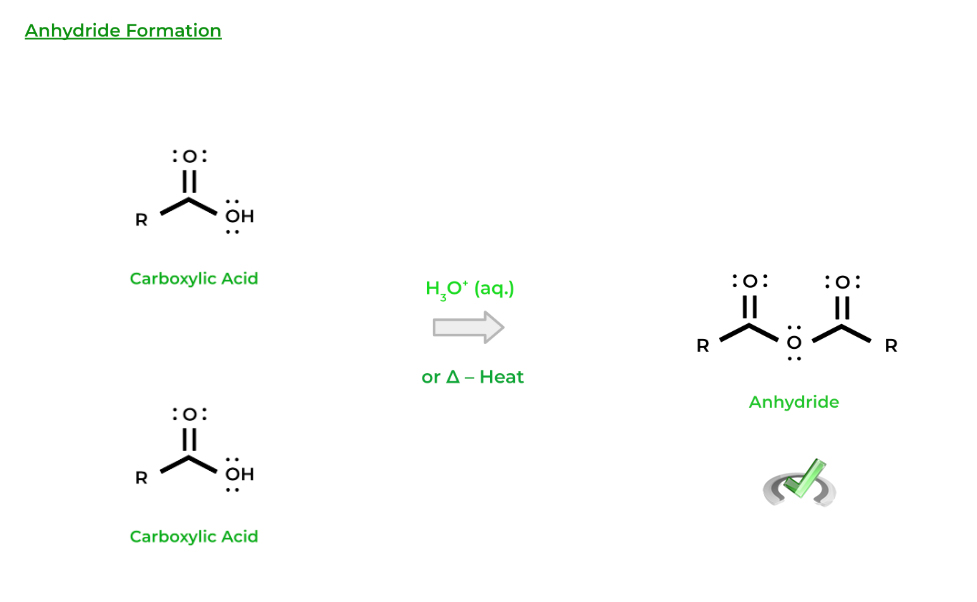
Note that all of the resulting products are carboxylic acid derivatives — we’ll get more into these derivatives in the next coming sections!
Full Study Notes : Nucleophilic substitution reactions of carboxylic acids
For more in-depth content review on nucleophilic substitution reactions of carboxylic acids, check out these detailed lesson notes created by top MCAT scorers.
3. Other Reactions of Carboxylic Acids
Aside from the nucleophilic substitution reactions, there are other reactions that carboxylic acids can participate in! While carboxylic acids can participate in redox reactions, we’ll primarily cover saponification and decarboxylation reactions — check out our other chapter overviews which cover the redox reactions of carboxylic acids!
The saponification of carboxylic acids basically refers to the formation of a salt! When treated with another salt with a strong base such as sodium or potassium hydroxide, the hydroxide ion can deprotonate the acidic hydrogen of the hydroxyl group.
This leaves a negatively charged oxygen atom which forms favorable electrostatic interactions with the positively charged sodium cation. This is often done with fatty acids, which are carboxylic acids with long carbon chains!
Another reaction that carboxylic acids participate in are decarboxylation reactions! As the name suggests, we lose a carboxyl group in the form of carbon dioxide. These reactions occur a lot in many reactions in oxidative phosphorylation.
The key concept to note with decarboxylation reactions is that it can ONLY occur with 1,3-dicarboxylic acids and 𝜷-keto acids. The reasoning for this will be further explained in our specialized article — for now just commit this concept to memory!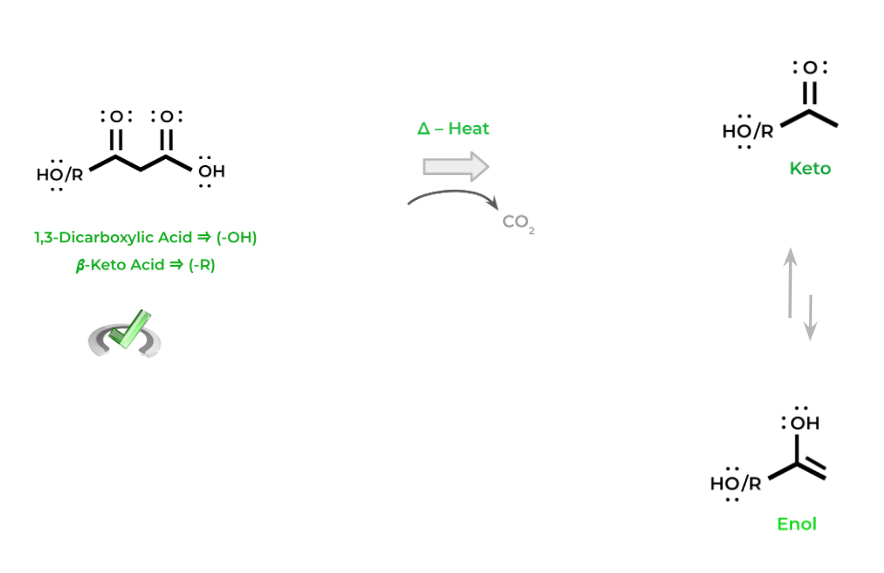
You’ll notice that the resulting products participate in keto-enol tautomerism – this is because the intermediate formed from the decarboxylation is an enolate which can tautomerism between the keto and enol form.
Full Study Notes : Additional reactions of carboxylic acids
For more in-depth content review on additional reactions of carboxylic acids, check out these detailed lesson notes created by top MCAT scorers.
4. Basic Structure and Nomenclature of Carboxylic Acid Derivatives
Earlier, we covered 3 main carboxylic acids derivatives — let’s now go over their basic nomenclature! Before getting into their nomenclature, we want to preface that we’ll only cover cases when the derivatives are the highest priority functional group!
There are definitely cases when the derivatives are not the highest priority functional groups, but generally, they are very low yield. You can find the prefixes with a quick search online, but what’s covered here should suffice!
Just like when naming other molecules, base it off of the corresponding alkane indicated by the number of carbons! For amides, simply replace the “-e” suffix with “-amide”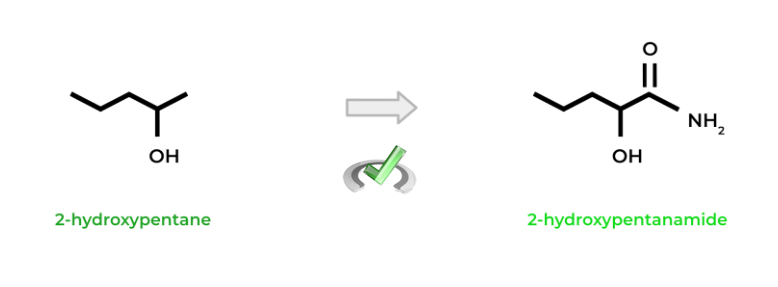
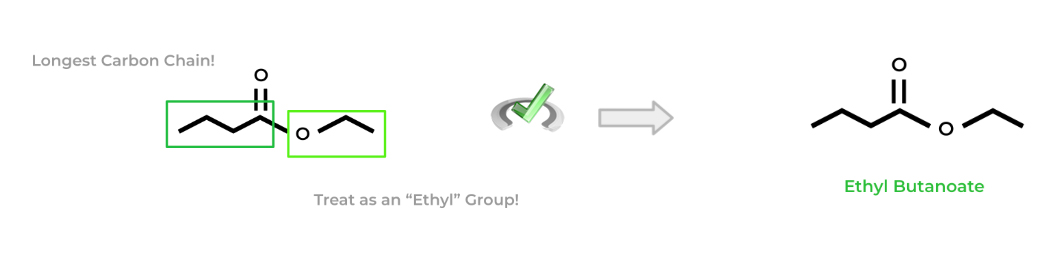
The naming of esters is somewhat similar to naming ethers! First, find the longest carbon chain containing the carbonyl carbon. Then, treat the ester group kinda like an alkyl group by looking at the number of carbons present. Finally, your parent chain will replace the “-e” suffix from the corresponding alkane with “-oate”.
Anhydride nomenclature is somewhat similar to ester nomenclature but has one distinct difference. Anhydrides can be classified as either symmetric or asymmetric anhydrides depending on the number of carbons on each side of the functional group.
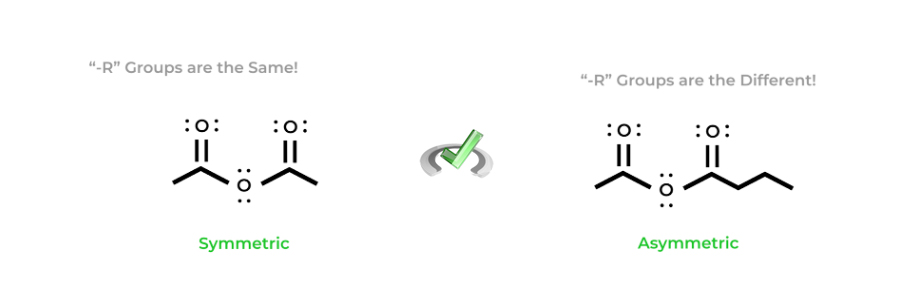
In anhydride nomenclature, we’ll actually use the naming of carboxylic acids as a basis. All we really do is replace the “-acid” with “-anhydride” — we just have to be careful and identify whether the anhydride is symmetric or asymmetric!
If the anhydride is symmetric, simply state the name of the alkyl R group. If the anhydride is asymmetric, you must name the R groups in alphabetical order. Remember to also utilize the “-oic” suffix for the R groups! It’s a lot easier to understand with a visual example below as reading it is a bit confusing!
Full Study Notes : The nomenclature of these 3 main carboxylic acid derivatives
For more in-depth content review on the nomenclature of these 3 main carboxylic acid derivatives, check out these detailed lesson notes created by top MCAT scorers.
5. Nucleophilic Substitution Reactions of Carboxylic Acid Derivatives
Because these carboxylic acid derivatives also have carbonyl chemistry, they can function in nucleophilic substitution reactions! The 2 main reactions that we’ll focus on are hydrolysis and transesterification.
We can use a water molecule to “lyse” any of the carboxylic acid derivatives back into a carboxylic acid(s) — hence the term hydrolysis. Just like some of the other organic chemical reactions we’ve covered, hydrolysis can be acid or base catalyzed.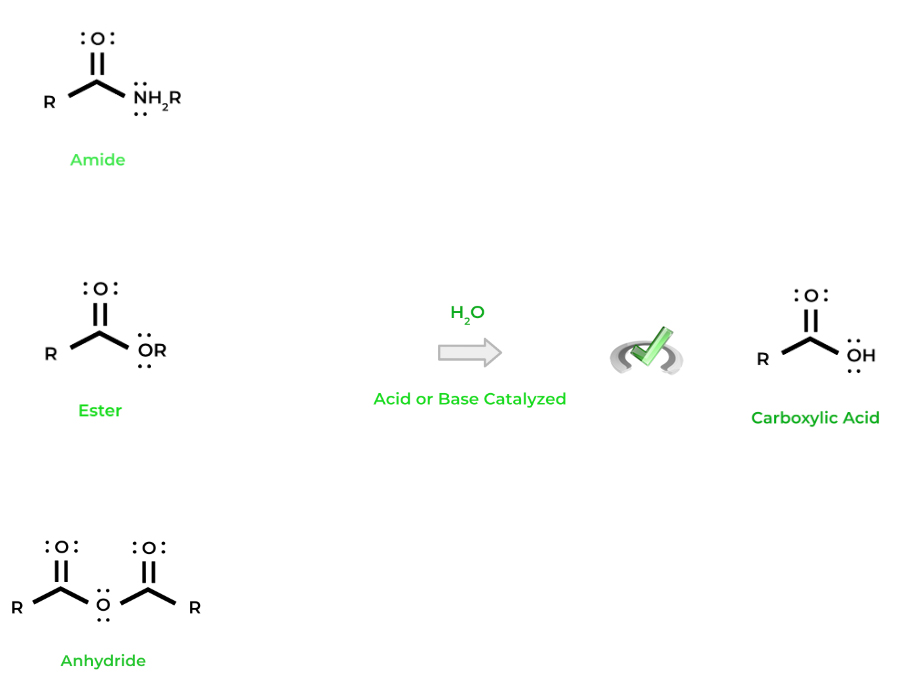
Finally, transesterification occurs when one ester is converted into another ester! In this reaction, a hydroxyl group acts as a nucleophile and kicks off the prior esterifying group on the ester. Most of the time, the reaction is acid catalyzed similar to the formation of an ester from a carboxylic acid!
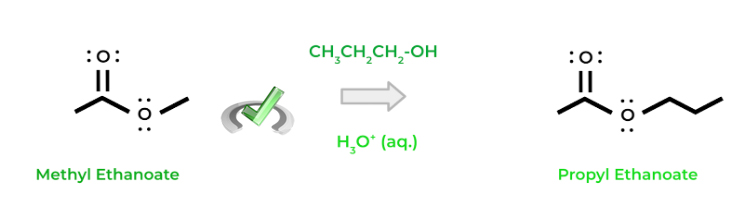
Full Study Notes : Different nucleophilic substitution reactions of carboxylic acid derivatives
For more in-depth content review on different nucleophilic substitution reactions of carboxylic acid derivatives, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about carboxylic acids and their derivatives in organic chemistry!
Term | Definition |
|---|---|
Carboxylic Acid | An organic molecule characterized by the presence of a carboxyl group which is composed of a carbonyl group with a hydroxyl group attached to the carbonyl carbon |
Ester | Carboxylic acid derivative formed by the nucleophilic substitution of an alcohol on a carboxylic acid |
Amide | Carboxylic acid derivative formed by the nucleophilic substitution of an amine on a carboxylic acid |
Anhydride | Carboxylic acid derivative formed by the nucleophilic substitution of the hydroxyl on one carboxylic acid on another carboxylic acid |
Saponification | A type of reaction which results in the formation of a salt; Often occurs with the addition of a hydroxide salt with a fatty acid |
Decarboxylation | A reaction that results in the loss of a carboxyl groups in the form of a carbon dioxide; Primarily occurs in 𝜷-keto acids and 1,3-dicarboxylic acids |
Hydrolysis | The lysis of molecules via the nucleophilic substitution of water which can be acid/base catalyzed |
Transesterification | Reaction where the esterifying group of one ester is substituted for another esterifying group |
Additional FAQs - Aldehydes and Ketones on the MCAT
What Are 3 Examples of Carboxylic Acids? – MCAT
What is the Inductive Effect? – MCAT
The acidity of the hydroxyl hydrogen of carboxylic acids can also be attributed to the stabilization of the charge upon deprotonation. The resonance stabilization of the negative charge increases the stability of the conjugate base!
What are the Derivatives of Carboxylic Acids? – MCAT
How Can You Tell if a Molecule is a Carboxylic Acid Derivative? – MCAT
How Are Carboxylic Acids Converted into their Derivatives? – MCAT
Do You Need to Know Orgo Mechanism for the MCAT? – MCAT
Remember, the MCAT is still multiple choice so just knowing the superficial generalities of a nucleophilic substitution reaction, for example, will be good enough for your success on the MCAT!
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Alcohols and Ethers on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 




















