Perhaps in your introductory biology classes in college (or even high school!), you may have been taught the mnemonic CHON, which refers to the most common elements found in organic compounds – carbon, hydrogen, oxygen, and nitrogen!
It comes as no surprise that a lot of the biochemical molecules and compounds that’ll be covered in your MCAT prep contain nitrogen! From amino acids to the nitrogenous bases that make up the DNA nucleotides, they are a crucial set of molecules for our human body!
In this chapter overview, you’ll get a brief overview of some of the important nitrogen containing compounds and some of their associated processes. Let’s dive in!
Nitrogen Containing Compounds on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Similar to our phosphorus containing compounds section, this section is not as highly tested as compared to other organic chemistry topics. Instead of expecting a certain number of questions, take the concepts covered from this chapter overview and apply them when learning other MCAT topics, especially in biochemistry!
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Nitrogen Containing Compounds
A lot of the topics covered in this chapter overview may be review to you or look familiar! Take this opportunity to brush up on some of these topics!
Amino Acids and Nucleic Acid Bases
The obvious nitrogen-containing compounds within our human physiology are amino acids, as these are the monomers composing the polypeptide chains of proteins! As such, it comes as no surprise that they have such importance in our biochemistry!
There are 20 proteinogenic amino acids, meaning they can be linked together to make the polypeptide chains of proteins. Furthermore, the amino acids can also be categorized based on their shared characteristics.
Each of the amino acids has a common structure shown below with 3 main components: an amino group (-NH2), a carboxyl group (-COOH), and a side chain (- A). All of these are connected to a central, alpha carbon – recall that the 𝜶-carbon is the carbon adjacent to the carbonyl carbon.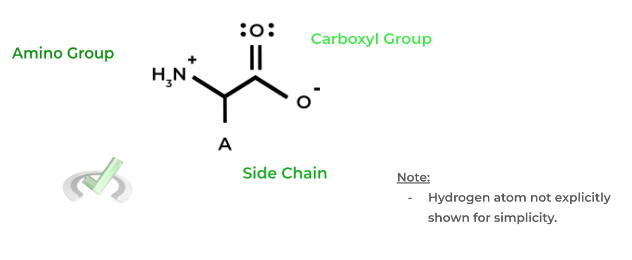
Note that the protonation states of the amino and carboxyl group shown above are for when the amino acid is in physiological pH (approx. 7.4). It follows that the carboxyl group has a much lower pKa than the amino group – 2.3 and 9.6, respectively.
The importance of amino acids as nitrogen containing compounds, especially in the context of organic chemistry, is seen during peptide bond formation between 2 amino acids!
The nitrogen-containing amino group goes on to function as a nucleophile and attack the carbonyl carbon of another amino acid. This results in a nucleophilic substitution reaction and ultimately results in the formation of a peptide bond!
Specifically, this reaction is called a condensation reaction because it results in a release of water.
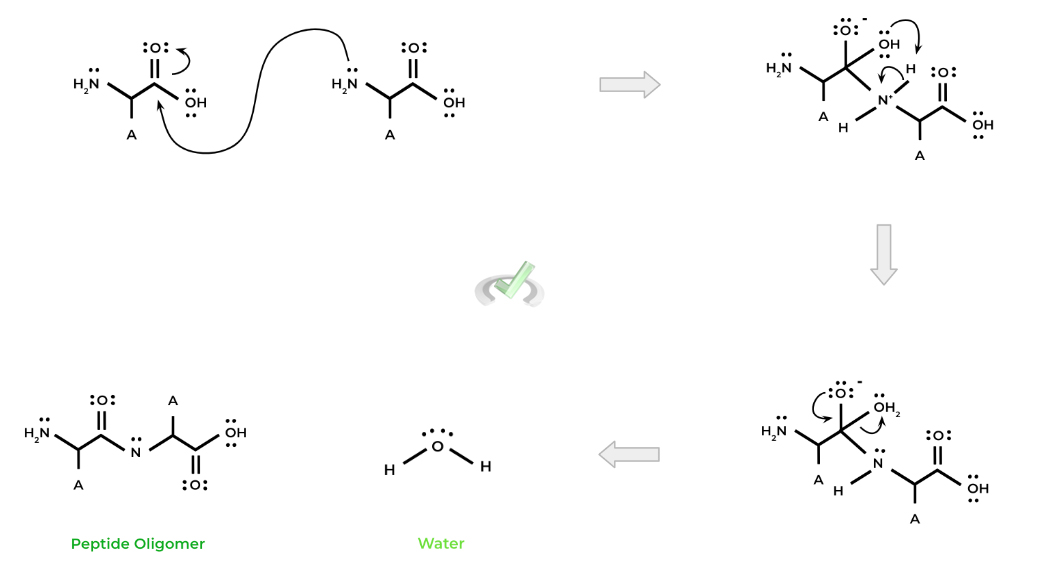
Another important nitrogen containing compound that you’ll come across is the nitrogenous bases that are a crucial component of the monomers of DNA polynucleotide!
Recall that the nitrogenous bases can be categorized into purines and pyrimidines. Purines, which include adenine and guanine, are 2 ringed bases, while pyrimidines, which include cytosine, thymine, and uracil, are single ringed as shown below!
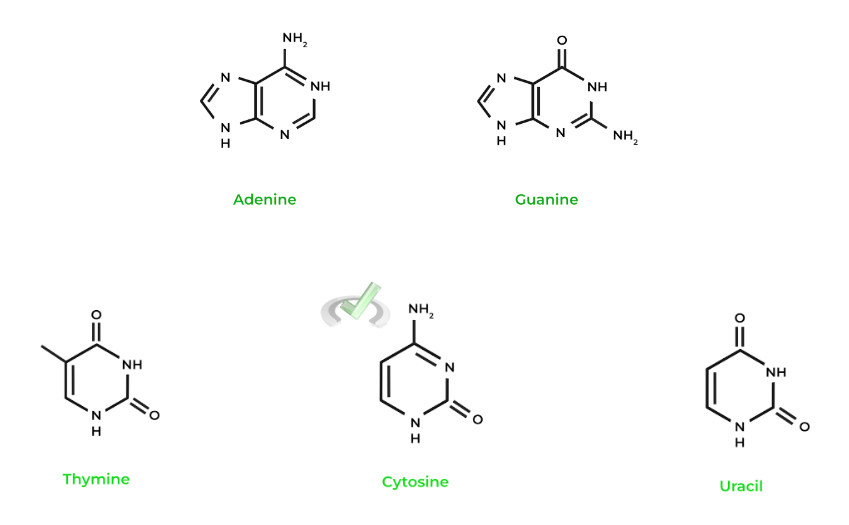
An important function of the nitrogen functional groups that are present in the bases is the hydrogen bonding they participate in with other complementary bases! Recall that the hydrogen bonding that exists between the nucleotides stabilized the double helix of the DNA.
(Coming Soon!) Full Study Notes : Amino Acids And Nucleic Acid Bases
For more on amino acids and nucleic acid bases as well as their main characteristics, check out these detailed lesson notes created by top MCAT scorers.
Synthesis of Amino Acids
Sometimes, scientists and researchers need to synthesize amino acids in vivo for study in a laboratory setting. Luckily, there are many different techniques that can be used to help us synthesize these amino acids!
Before getting into the 2 methods we’ll discuss, we want to emphasize that you should not focus on the mechanisms for these methods. Instead, get comfortable with seeing the reactants and conditions for each reaction and understanding that they result in the formation of an amino acid.
One method for amino acid synthesis is the Streckter synthesis, which involves 3 main steps as shown below. The molecules in parenthesis just say what common molecules are used in each step.3 Main Steps for Strecker Synthesis
A. Imine Formation from Aldehyde ⇒ (:NH3-)
B. Cyanide Addition to Imine ⇒ (K+ CN-)
C. Acid Hydrolysis ⇒ (H3O+, aqueous)
An important concept to note is that an aldehyde must be used! When the reaction is complete, we see that the carbonyl carbon of the aldehyde will actually become the 𝜶-carbon of the amino acid! Below is a common setup that you might see!
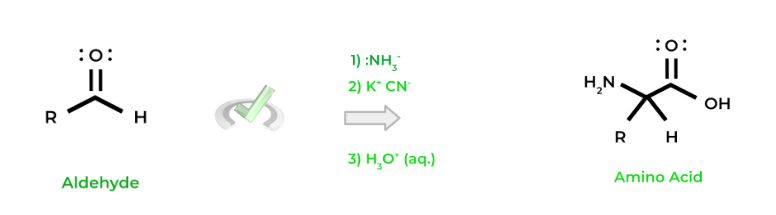
Finally, one more thing to take note of is that the R group on the aldehyde will determine the amino acid that’s created (i.e. valine, tryptophan, etc.). You’ll most likely be asked, given the aldehyde and the reactants for Strecker synthesis, what amino acid will form and/or its properties!
Another, though more complicated, procedure is the Gabriel synthesis. In addition to making amino acids, primary amines can also be synthesized via this process. The Gabriel synthesis involves 4 main steps as shown below:
4 Main Steps for Gabriel Synthesis
A. Deprotonation of N-Phthalimidomalonic Ester By Strong Base⇒ (Na+ OEth-)
B. SN2 Reaction with Alkyl Halide ⇒ (R-Br)
C. Acid Hydrolysis ⇒ (H3O+, aqueous)
D. Decarboxylation ⇒ (Heat – 𝚫)
The most important concept to understand from the Gabriel synthesis is that the side chain of the amino acid will come from the R group attached to the alkyl halide, similar to the R group on the aldehyde in the Strecker synthesis. Look at a common setup below!
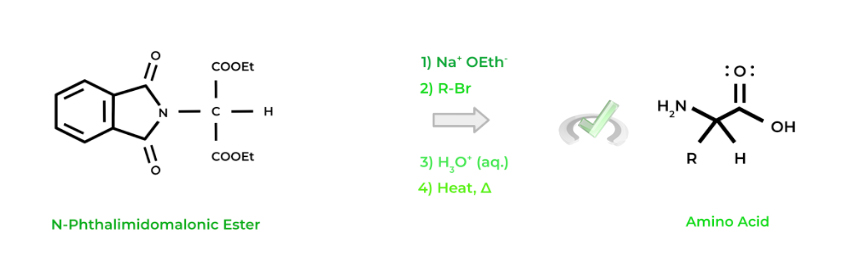
Similar to the Streker synthesis, get comfortable with identifying the reactants that are necessary for Gabriel synthesis and identify what amino acid will form based on the alkyl halide!
(Coming Soon!) Full Study Notes : Strecker and Gabriel synthesis of amino acids
For more on Strecker and Gabriel synthesis of amino acids, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about nitrogen containing compounds in organic chemistry!
Term | Definition |
|---|---|
Amino Acid | Monomers that make up the backbone of a protein’s polypeptide chain; Contain an amino group (-NH2), carboxyl group (-COOH), and a side chain (-A) which are all connected to a side chain |
Purines | 2 ringed nitrogenous bases; adenine and guanine are classified as purines |
Pyrimidines | Single ring nitrogenous bases; thymine, cytosine, and uracil are classified as pyrimidines |
Strecker Synthesis | One method of synthesizing amino acids utilizing an aldehyde as the main starting reactant; “R” group of aldehyde determines the side chain of the formed amino acid |
Gabriel Synthesis | One method of synthesizing amino acids utilizing N-phthalimidomalonic ester as the main starting reactant; the alkyl group of the alkyl halide determines the side chain of the formed amino acid |
Additional Reading -- Organic Chemistry Topics:
- Aldehydes and Ketones on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Alcohols and Ethers on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT


 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these